IRB – Antibody Discovery
The antibody discovery and engineering program at the Institute for Research in Biomedicine (IRB)
Over two decades, the antibody discovery program at the IRB contributed to advances in monoclonal antibody technologies while making important scientific discoveries in the field of antibodies against a broad range of infectious diseases, such as those caused by coronaviruses, flaviviruses, respiratory syncytial virus, paramyxoviruses, influenza, rabies, parainfluenza and malaria (see references below).
Monoclonal antibodies that were discovered at the IRB, or using IRB-licensed technologies, are undergoing clinical development (for example CoV-X4042, which is effective against all coronavirus variants; Bianchini et al) and in some cases already reached clinical approval (e.g. Ansuvimab against ebola and Sotrovimab against COVID-19).
In recent years, the platform was expanded to include antibody engineering and computational modeling methods to enhance the effectiveness of natural antibodies (e.g. Bispecific IgG neutralizes SARS-CoV-2 variants and prevents escape in mice; De Gasparo et al ). Moreover, novel areas of endeavor include epitope-targeted discovery (e.g. Antibodies targeting highly conserved viral regions; Bianchini et al) and Discovery of post-infectious autoantibodies with anti-inflammatory potential; (Muri et al).
The antibody discovery and engineering activities at the IRB are in coordination with numerous international collaborators, including European consortia (e.g. Antibody Therapy Against Coronavirus, ATAC and Integrated Services for Infectious Disease Outbreak Research ISIDORe) and investigators in the United States and other Countries (United World Antiviral Research Network UWARN).
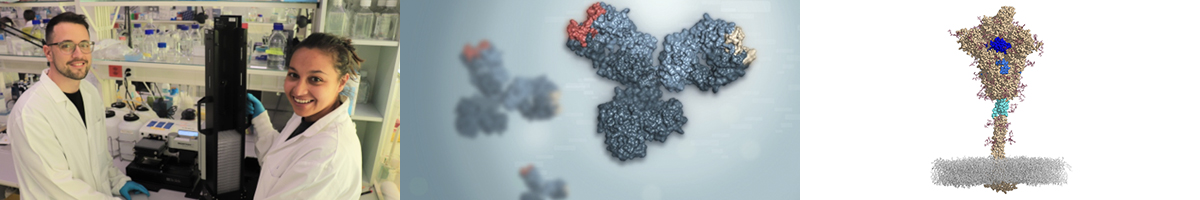
Left: Scientists in the Robbiani Lab characterizing anti-inflammatory antibodies from COVID-19 patients. Middle: Artistic rendering of a bispecific antibody against COVID-19 (CoV-X4042) currently undergoing clinical development (courtesy of Varani lab) Right: Structure of the coronavirus (SARS-CoV-2) spike molecule with highlighted in blue higly conserved regions targeted by virus-neutralizing “coldspot” antibodies (couresy of Cavalli lab).
Publications
Bianchini, F., Crivelli, V., Abernathy, M.E., Guerra, C., Palus, M., Muri, J., Marcotte, H., Piralla, A., Pedotti, M., De Gasparo, R., Simonelli, L., Matkovic, M., Toscano, C., Biggiogero, M., Calvaruso, V., Svoboda, P., Cervantes Rincón, T., Fava, T., Podešvová, L., Shanbhag, A.A., Celoria, A., Sgrignani, J., Stefanik, M., Hönig, V., Pranclova, V., Michalcikova, T., Prochazka, J., Guerrini, G., Mehn, D., Ciabattini, A., Abolhassani, H., Jarrossay, D., Uguccioni, M., Medaglini, D., Pan-Hammarström, Q., Calzolai, L., Fernandez, D., Baldanti, F., Franzetti-Pellanda, A., Garzoni, C., Sedlacek, R., Ruzek, D., Varani, L., Cavalli, A., Barnes, C.O. and D.F. Robbiani. Sci Immunol. 2023; 8(81):eade0958
Autoantibodies against chemokines post-SARS-CoV-2 infection correlate with disease course
Muri, J., V. Cecchinato, A. Cavalli, A. A. Shanbhag, M. Matkovic, M. Biggiogero, P. A. Maida, J. Moritz, C. Toscano, E. Ghovehoud, R. Furlan, F. Barbic, A. Voza, G. De Nadai, C. Cervia, Y. Zurbuchen, P. Taeschler, L. A. Murray, G. Danelon-Sargenti, S. Moro, T. Gong, P. Piffaretti, F. Bianchini, V. Crivelli, L. Podesvova, M. Pedotti, D. Jarrossay, J. Sgrignani, S. Thelen, M. Uhr, E. Bernasconi, A. Rauch, A. Manzo, A. Ciurea, M. B. L. Rocchi, L. Varani, B. Moser, B. Bottazzi, M. Thelen, B. A. Fallon, O. Boyman, A. Mantovani, C. Garzoni, A. Franzetti-Pellanda, M. Uguccioni and D. F. Robbiani. Nat Immunol. 2023; 24:604-611.
Broad and potent neutralizing human antibodies to tick-borne flaviviruses protect mice from disease
Agudelo, M., M. Palus, J. R. Keeffe, F. Bianchini, P. Svoboda, J. Salat, A. Peace, A. Gazumyan, M. Cipolla, T. Kapoor, F. Guidetti, K. H. Yao, J. Elsterova, D. Teislerova, A. Chrdle, V. Honig, T. Oliveira, A. P. West, Y. E. Lee, C. M. Rice, M. R. MacDonald, P. J. Bjorkman, D. Ruzek, D. F. Robbiani and M. C. Nussenzweig. J Exp Med. 2021; 218:e20210236
Structural basis of malaria RIFIN binding by LILRB1-containing antibodies
Chen, Y., K. Xu, L. Piccoli, M. Foglierini, J. Tan, W. Jin, J. Gorman, Y. Tsybovsky, B. Zhang, B. Traore, C. Silacci-Fregni, C. Daubenberger, P. D. Crompton, R. Geiger, F. Sallusto, P. D. Kwong and A. Lanzavecchia. Nature. 2021; 592:639-643
Bispecific IgG neutralizes SARS-CoV-2 variants and prevents escape in mice
De Gasparo, R., M. Pedotti, L. Simonelli, P. Nickl, F. Muecksch, J. C. C. Lorenzi, F. Mazzola, D. Magrì, T. Michalcikova, J. Haviernik, V. Honig, I. Cassaniti, E. Percivalle, B. Mrazkova, N. Polakova, A. Fortova, J. Tureckova, V. Iatsiuk, S. Di Girolamo, P. Palus, D. Zudova, P. Bednar, I. Bukova, F. Bianchini, D. Mehn, R. Nencka, P. Strakova, O. Pavlis, J. Rozman, S. Gioria, J. C. Sammartino, F. Giardina, S. Gaiarsa, Q. P. Hammarström, C. O. Barnes, A. Piralla, P. J. Bjorkman, F. Baldanti, L. Calzolai, M. C. Nussenzweig, P. D. Bieniasz, T. Hatziioannou, J. Prochazka, R. Sedlacek, D. F. Robbiani, D. Ruzek and L. Varani. Nature. 2021; 593:424-428.
Cheung, C. S., A. Fruehwirth, P. C. G. Paparoditis, C. H. Shen, M. Foglierini, M. G. Joyce, K. Leung, L. Piccoli, R. Rawi, C. Silacci-Fregni, Y. Tsybovsky, R. Verardi, L. Wang, S. Wang, E. S. Yang, B. Zhang, Y. Zhang, G. Y. Chuang, D. Corti, J. R. Mascola, L. Shapiro, P. D. Kwong, A. Lanzavecchia and T. Zhou. Cell Rep. 2020; 32:108088.
Marcandalli, J., B. Fiala, S. Ols, M. Perotti, W. de van der Schueren, J. Snijder, E. Hodge, M. Benhaim, R. Ravichandran, L. Carter, W. Sheffler, L. Brunner, M. Lawrenz, P. Dubois, A. Lanzavecchia, F. Sallusto, K. K. Lee, D. Veesler, C. E. Correnti, L. J. Stewart, D. Baker, K. Lore, L. Perez and N. P. King. Cell. 2019; 176(6): 1420-1431 e1417
Lee, J., P. Paparoditis, A. P. Horton, A. Fruhwirth, J. R. McDaniel, J. Jung, D. R. Boutz, D. A. Hussein, Y. Tanno, L. Pappas, G. C. Ippolito, D. Corti, A. Lanzavecchia and G. Georgiou. Cell Host Microbe. 2019; 25(3): 367-376 e365
Stewart-Jones, G. B. E., G. Y. Chuang, K. Xu, T. Zhou, P. Acharya, Y. Tsybovsky, L. Ou, B. Zhang, B. Fernandez-Rodriguez, V. Gilardi, C. Silacci-Fregni, M. Beltramello, U. Baxa, A. Druz, W. P. Kong, P. V. Thomas, Y. Yang, K. E. Foulds, J. P. Todd, H. Wei, A. M. Salazar, D. G. Scorpio, B. Carragher, C. S. Potter, D. Corti, J. R. Mascola, A. Lanzavecchia and P. D. Kwong. Cell Host Microbe. 2019; 25(3): 367-376 e365
Tan, J., B. K. Sack, D. Oyen, I. Zenklusen, L. Piccoli, S. Barbieri, M. Foglierini, C. S. Fregni, J. Marcandalli, S. Jongo, S. Abdulla, L. Perez, G. Corradin, L. Varani, F. Sallusto, B. K. L. Sim, S. L. Hoffman, S. H. I. Kappe, C. Daubenberger, I. A. Wilson and A. Lanzavecchia. Net Med. 2018; 24: 401-407
Public antibodies to malaria antigens generated by two LAIR1 insertion modalities
Pieper, K., J. Tan, L. Piccoli, M. Foglierini, S. Barbieri, Y. Chen, C. Silacci-Fregni, T. Wolf, D. Jarrossay, M. Anderle, A. Abdi, F. M. Ndungu, O. K. Doumbo, B. Traore, T. M. Tran, S. Jongo, I. Zenklusen, P. D. Crompton, C. Daubenberger, P. C. Bull, F. Sallusto and A. Lanzavecchia. Nature. 2017; 548: 597-601
Antibody-guided vaccine design: identification of protective epitopes
Lanzavecchia A, Fruhwirth A, Perez L, and D. Corti. Curr Opin Immunol. 2016; 41: 62-7
Development of broad-spectrum human monoclonal antibodies for rabies post-exposure prophylaxis
De Benedictis P, Minola A, Rota Nodari E, Aiello R, Zecchin B, Salomoni A, Foglierini M, Agatic G, Vanzetta F, Lavenir R, Lepelletier A, Bentley E, Weiss R, Cattoli G, Capua I, Sallusto F, Wright E, Lanzavecchia A, Bourhy H, and D. Corti. EMBO Mol Med. 2016; 8: 407-21
A LAIR1 insertion generates broadly reactive antibodies against malaria variant antigens
Tan J, Pieper K, Piccoli L, Abdi A, Foglierini M, Geiger R, Tully CM, Jarrossay D, Ndungu FM, Wambua J, Bejon P, Fregni CS, Fernandez-Rodriguez B, Barbieri S, Bianchi S, Marsh K, Thathy V, Corti D, Sallusto F, Bull P, and A. Lanzavecchia. Nature. 2016; 529: 105-9
Corti D, Zhao J, Pedotti M, Simonelli L, Agnihothram S, Fett C, Fernandez-Rodriguez B, Foglierini M, Agatic G, Vanzetta F, Gopal R, Langrish CJ, Barrett NA, Sallusto F, Baric RS, Varani L, Zambon M, Perlman S, and A. Lanzavecchia. Proc Natl Acad USA 2015; 112: 10473-8
Cross-neutralization of four paramyxoviruses by a human monoclonal antibody
